Top 10 Healthcare Breakthroughs of 2025
Executive Strategic Overview: The Shift from Potential to Kinetics
The trajectory of healthcare innovation is rarely linear; it moves in distinct cycles of hypothesis, experimentation, and finally, realization. If the early 2020s were characterized by the rapid, emergency-driven innovation of the pandemic era, and 2023-2024 by the theoretical explosion of generative artificial intelligence and gene editing possibilities, 2025 stands as the Year of Realization.
In 2025, technologies that had existed primarily in white papers and Phase 1 safety trials crossed the threshold into clinical validity and commercial scalability. This year did not merely promise cures; it delivered them. The medical community witnessed the transition of “n=1” gene editing from a scientific curiosity to a regulatory precedent; the shift of AI from a backend administrative tool to a multimodal clinical partner; and the first genuine crack in the opioid crisis’s pharmacological foundation in two decades.
The retrospective analysis of 2025 reveals three macro-trends that define the current healthcare landscape:
- The Industrialization of Precision: The “blockbuster” model, reliant on one-size-fits-all drugs, has been definitively superseded by a “fragmentation” model. Whether in the treatment of Acute Myeloid Leukemia (AML) or Non-Small Cell Lung Cancer (NSCLC), success in 2025 was defined by targeting specific molecular signatures (e.g., NPM1 mutations, TROP2 expression) rather than broad anatomical indications.
- The Curative Economic Model: The healthcare system is beginning to grapple with the financial reality of cures. With the commercial scaling of sickle cell gene therapies and the advent of regenerative heart patches, the sector is moving from chronic disease management—paid for in installments over a lifetime—to high-value, one-time interventions. This shift was solidified in 2025 by new reimbursement frameworks in the US and UK that accommodate these high-upfront costs.
- Decentralization of Acuity: Care is leaving the hospital. Through breakthroughs in long-acting injectables (HIV PrEP), subcutaneous delivery devices (Lasix ONYU), and autonomous diagnostic kiosks (OnMed), high-acuity care is increasingly being delivered in the community setting, reducing the burden on centralized infrastructure.
This report provides an exhaustive analysis of the top ten breakthroughs of 2025. These selections are derived from an evaluation of clinical trial data, regulatory approvals (FDA/EMA), peer-reviewed publications (Nature, NEJM), and their demonstrated impact on patient morbidity and mortality.
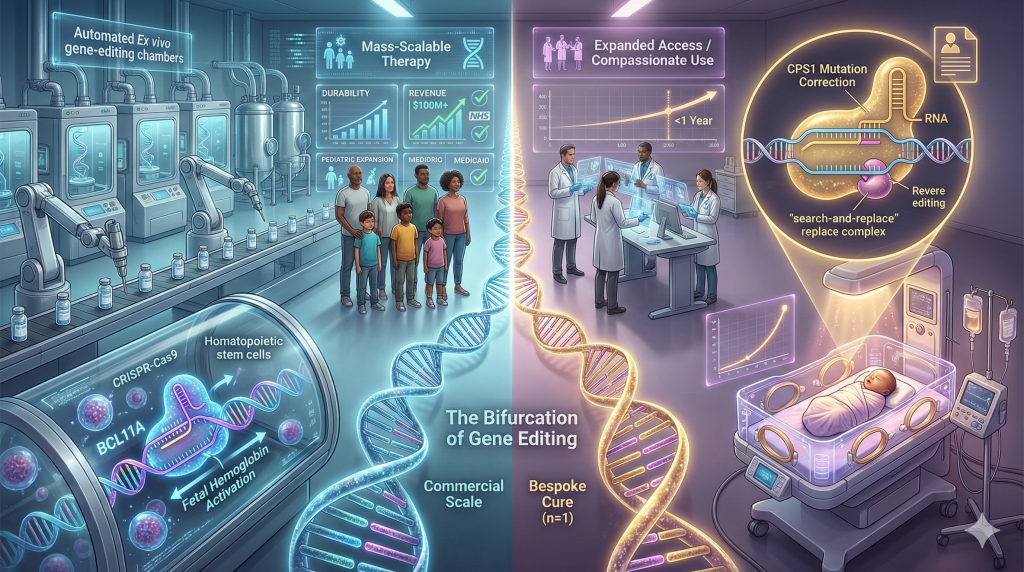
1. The Bifurcation of Gene Editing: Commercial Scale and Bespoke Cures
The narrative of gene editing in 2025 is a story of two diverging but complementary paths: the successful scaling of approved therapies for rare diseases and the unprecedented regulatory flexibility granted to hyper-personalized treatments for unique genetic errors.
The Commercial Maturation of Casgevy
By late 2025, Casgevy (exagamglogene autotemcel), the first CRISPR/Cas9-based therapy, had transitioned from a newly approved scientific marvel to a standard-of-care option for Sickle Cell Disease (SCD) and Transfusion-Dependent Beta Thalassemia (TDT). Developed by Vertex Pharmaceuticals and CRISPR Therapeutics, Casgevy functions by editing the BCL11A gene in a patient’s hematopoietic stem cells to reactivate fetal hemoglobin production.1
Clinical and Commercial Velocity:
Data presented in December 2025 confirmed that the therapy’s efficacy is durable and replicable across broader demographics.
- Pediatric Expansion: Critical trials concluded in 2025 demonstrated efficacy in children aged 5 to 11 years. Treating this demographic is strategically vital, as it allows for the prevention of the cumulative organ damage (stroke, splenic infarction) that characterizes adult SCD.1
- Revenue and Access: Vertex reported over $100 million in revenue for Casgevy in 2025 alone, a figure that validates the scalability of the complex ex vivo manufacturing supply chain.3
- Reimbursement Stabilization: The signing of formal reimbursement agreements with NHS England and various US Medicaid programs in 2025 marked the stabilization of the economic model for gene therapy. These agreements signaled payer willingness to absorb high upfront costs (approx. $2.2 million) in exchange for the cessation of lifetime transfusion and hospitalization costs.4
The “Baby KJ” Precedent: The Era of Programmable Medicine
While Casgevy proved CRISPR could treat thousands, the case of “Baby KJ” proved it could be engineered for a single individual. In a landmark achievement for interventional genetics, researchers at the Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania utilized a bespoke CRISPR prime editor to treat a neonate with Carbamoyl Phosphate Synthetase 1 (CPS1) deficiency.5
The Clinical Challenge:
CPS1 deficiency is an ultra-rare urea cycle disorder leading to hyperammonemia, neurologic damage, and early death. Traditional drug development economics fail for such rare conditions, as the patient population is too small to recoup R&D costs.
The Breakthrough:
The therapy for Baby KJ was not a standard CRISPR “cut” but a prime editing approach—a “search-and-replace” technology that corrects the specific mutation without inducing double-strand breaks, thereby reducing the risk of off-target effects.6
- Timeline: The team moved from diagnosis to administration in under one year, utilizing an expanded access “compassionate use” pathway.7
- Regulatory Implication: This case established a framework for treating “n=1” diseases. In October 2025, researchers proposed a new clinical trial model where the delivery vehicle and enzyme are standardized (“the platform”), and only the guide RNA is customized for the patient. This “platform approval” concept could unlock treatments for thousands of ignored genetic diseases.9
| Feature | Casgevy (Standard) | Baby KJ Therapy (Bespoke) |
| Disease Target | Sickle Cell / Beta Thalassemia | CPS1 Deficiency |
| Target Population | >30,000 potential patients | 1 patient (n=1 model) |
| Editing Tech | CRISPR-Cas9 (Double-strand break) | Prime Editing (Precision rewrite) |
| Delivery | Ex vivo (cells edited in lab) | In vivo (systemic infusion) |
| Regulatory Path | Traditional BLA Approval | Compassionate Use / Platform Trial |
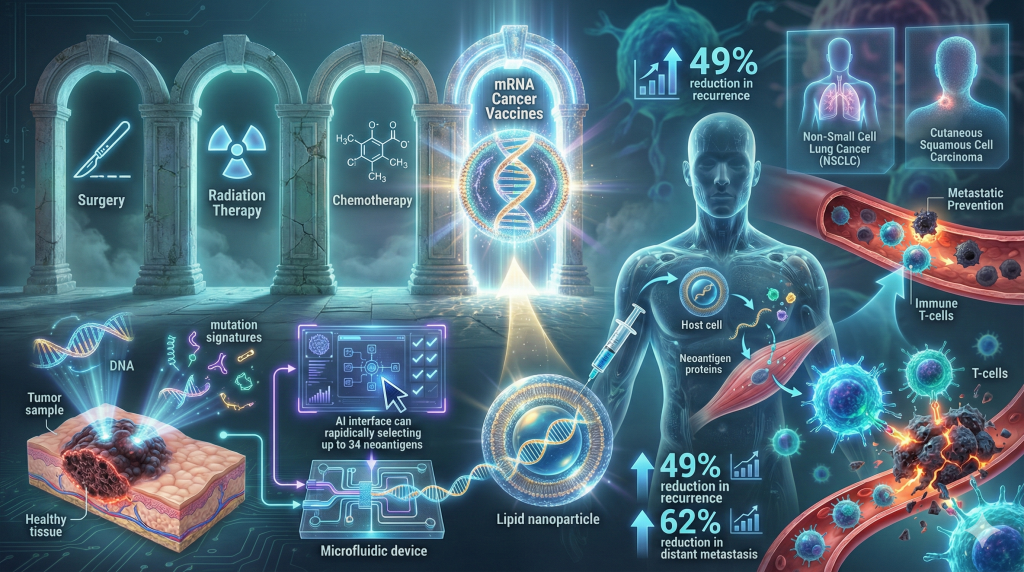
2. The Fourth Pillar of Oncology: mRNA Cancer Vaccines
Following the validation of mRNA technology during the COVID-19 pandemic, 2025 saw the technology successfully pivot to its original intended purpose: oncology. The most significant development was the release of long-term Phase 3 and Phase 2b data for mRNA-4157 (V940), a personalized neoantigen therapy developed by Moderna and Merck.10
Mechanism of Action: The Neoantigen Approach
Unlike off-the-shelf drugs, mRNA-4157 is manufactured on demand.
- Sequencing: A patient’s tumor and healthy tissue are sequenced to identify unique mutations (neoantigens) present only on the cancer cells.
- Algorithm Selection: An AI algorithm selects up to 34 neoantigens most likely to trigger a robust immune response.
- Synthesis: An mRNA strand encoding these antigens is synthesized and encapsulated in a lipid nanoparticle (LNP).
- Administration: When injected, the patient’s cells produce these antigens, training T-cells to hunt down and destroy cells bearing these specific mutational signatures.11
Clinical Efficacy in Melanoma
In 2025, data from the KEYNOTE-942 trial demonstrated that mRNA-4157, when combined with the checkpoint inhibitor pembrolizumab (Keytruda), fundamentally altered the trajectory of high-risk melanoma.
- Recurrence-Free Survival (RFS): The combination reduced the risk of recurrence or death by 49% compared to pembrolizumab alone.10
- Distant Metastasis-Free Survival (DMFS): Crucially, the risk of distant metastasis (cancer spreading to other organs) was reduced by 62%. This metric is the strongest indicator that the vaccine successfully generates systemic immune surveillance.10
The success in melanoma has catalyzed a rapid expansion of the platform. In 2025, trials were expanded to include Non-Small Cell Lung Cancer (NSCLC) and cutaneous squamous cell carcinoma, suggesting that mRNA vaccines will become the “fourth pillar” of cancer treatment, standing alongside surgery, radiation, and chemotherapy.13
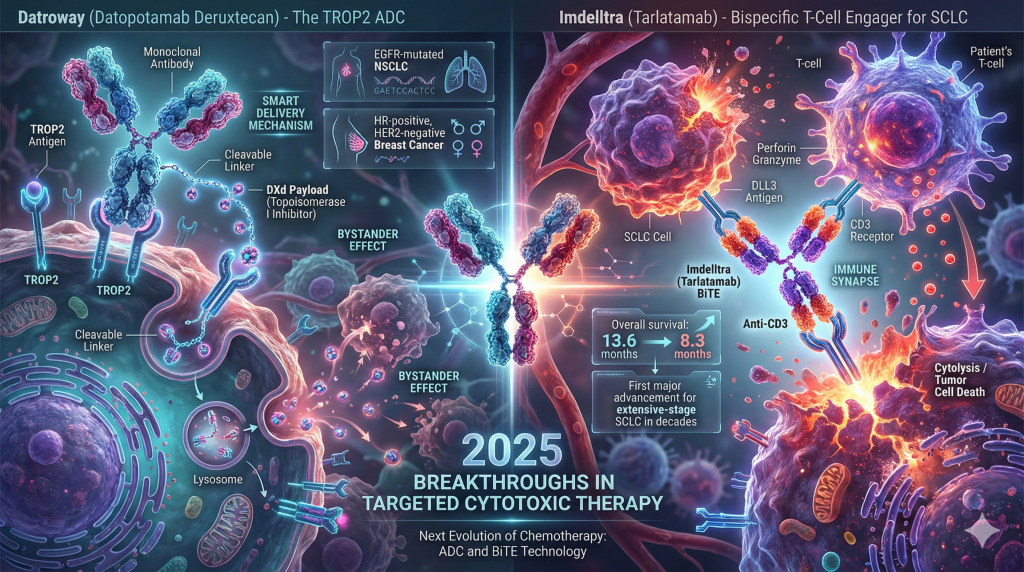
3. Precision Chemotherapy and Bispecifics: Reshaping Lung and Breast Cancer
While mRNA vaccines represent the future of immunotherapy, 2025 also delivered massive advancements in cytotoxic therapy through “smart delivery” systems: Antibody-Drug Conjugates (ADCs) and Bispecific T-cell Engagers (BiTEs).
Datroway (Datopotamab Deruxtecan): The TROP2 ADC
In 2025, the FDA granted approval to Datroway, a TROP2-directed ADC developed by Daiichi Sankyo and AstraZeneca, for specific subsets of lung and breast cancer.15
- The TROP2 Target: Trophoblast cell-surface antigen 2 (TROP2) is a transmembrane glycoprotein overexpressed in the majority of non-small cell lung cancers (NSCLC) and breast cancers.
- The DXd Payload: Datroway utilizes a proprietary topoisomerase I inhibitor payload connected via a cleavable linker. This design allows for a potent “bystander effect,” where the drug kills the targeted cancer cell and then diffuses to kill neighboring tumor cells, overcoming the challenge of tumor heterogeneity.17
2025 Approved Indications:
- NSCLC: Approved in June 2025 for locally advanced or metastatic EGFR-mutated NSCLC in patients who have progressed on prior EGFR-directed therapy (like osimertinib) and platinum chemotherapy.16 This provides a critical lifeline for patients who have exhausted targeted options.
- Breast Cancer: Approved in January 2025 for HR-positive, HER2-negative metastatic breast cancer.15
Imdelltra (Tarlatamab): Breaking the SCLC Stagnation
Small Cell Lung Cancer (SCLC) has seen minimal therapeutic progress for decades. In November 2025, Amgen received full FDA approval for Imdelltra (tarlatamab), a bispecific T-cell engager (BiTE) for extensive-stage SCLC.20
- Mechanism: Imdelltra acts as a bridge. One arm of the molecule binds to DLL3 (a protein highly expressed on SCLC cells but rare on healthy tissue), and the other binds to CD3 on the patient’s T-cells. This forces the immune cell into direct contact with the cancer cell, inducing cytolysis.22
- Survival Benefit: The Phase 3 DeLLphi-304 study showed Imdelltra improved median overall survival to 13.6 months versus 8.3 months with standard chemotherapy, a massive improvement in this aggressive disease context.21
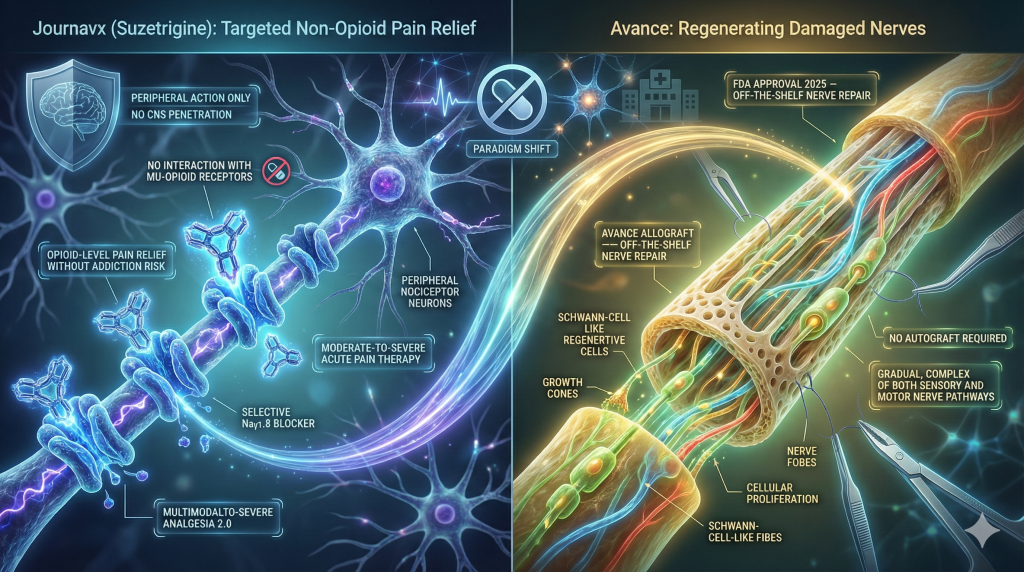
4. The Non-Opioid Pain Revolution: Journavx and Avance
The opioid crisis has been driven, in part, by a lack of effective alternatives for acute pain. 2025 marked the end of this pharmacological stagnation with the approval of a new class of analgesics.
Journavx (Suzetrigine): The NaV1.8 Inhibitor
In January 2025, the FDA approved Journavx (suzetrigine), developed by Vertex Pharmaceuticals, for moderate-to-severe acute pain.24
- Mechanism: Journavx is a selective inhibitor of NaV1.8, a voltage-gated sodium channel located specifically on peripheral nociceptors (pain-sensing neurons). Unlike opioids, it does not cross the blood-brain barrier to bind mu-opioid receptors in the central nervous system.24
- Clinical Impact: Trials confirmed that suzetrigine provides pain relief comparable to hydrocodone/acetaminophen combinations following surgeries like abdominoplasty, but with zero risk of physical dependence or addiction.24
- Systemic Shift: The approval has triggered a shift in hospital protocols dubbed “Multimodal 2.0,” where Journavx serves as the foundational analgesic, relegating opioids to rescue medication status only.25
Avance: Regenerating Nerves
Complementing the management of pain is the repair of the nerves themselves. In December 2025, the FDA approved Avance (acellular nerve allograft) for the surgical repair of peripheral nerve discontinuities.26 This off-the-shelf structural scaffold allows for the regeneration of sensory and motor nerves without the need to harvest a nerve from elsewhere in the patient’s body (autograft), reducing surgical morbidity.
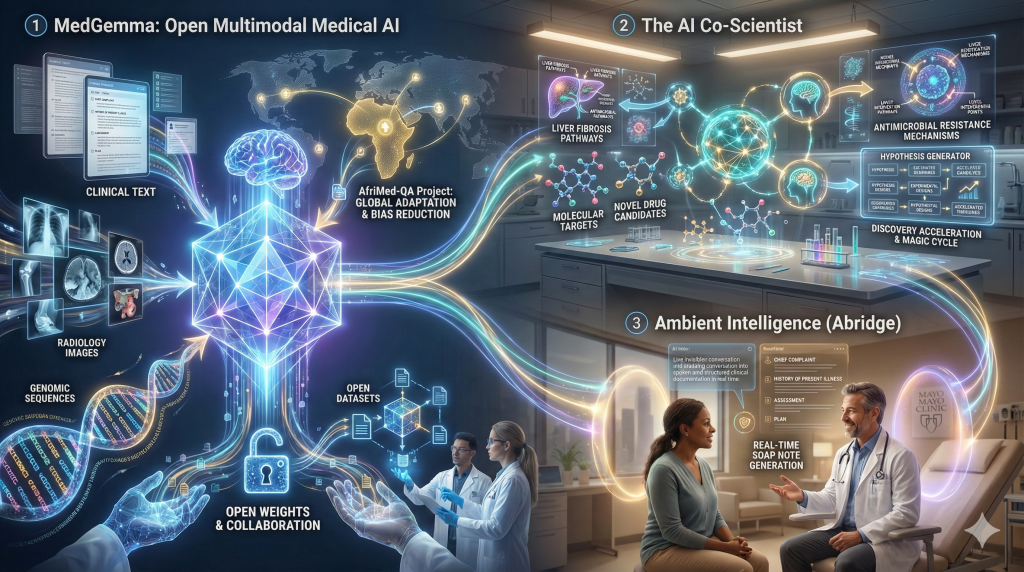
5. Artificial Intelligence: The Open Model & Ambient Era
In 2025, Artificial Intelligence in healthcare matured from a buzzword into a deployable infrastructure. The defining characteristic of AI in 2025 was the move toward Open Multimodal Models.
MedGemma: Democratizing Medical AI
Google released MedGemma in mid-2025, an open-weights foundation model built on the Gemini architecture.27
- Multimodality: MedGemma broke the text-only barrier. It was trained to simultaneously process clinical notes, radiology images (X-rays, CTs), and genomic data. This mimics the cognitive integration performed by a human clinician.28
- Open Architecture: By making the weights available, Google allowed researchers to fine-tune the model for specific local contexts. A prime example in 2025 was the AfriMed-QA project, where MedGemma was adapted to understand African medical contexts and healthcare delivery challenges, significantly reducing bias inherent in Western-trained models.30
- The “AI Co-Scientist”: Beyond clinical care, Google introduced the “AI Co-Scientist,” a multi-agent system based on Gemini 2.0. In 2025, this system demonstrated the ability to hypothesize novel drug targets for diseases like liver fibrosis and antimicrobial resistance, accelerating the “magic cycle” of discovery.31
Ambient Intelligence: Abridge
While MedGemma handled diagnostics, Abridge and similar “ambient” documentation platforms solved the administrative crisis. By late 2025, these systems—which listen to patient encounters and autonomously generate structured SOAP notes—were deployed at scale in systems like Mayo Clinic and Hartford HealthCare.32 This technology is widely credited with the first measured reduction in clinician burnout rates in a decade.
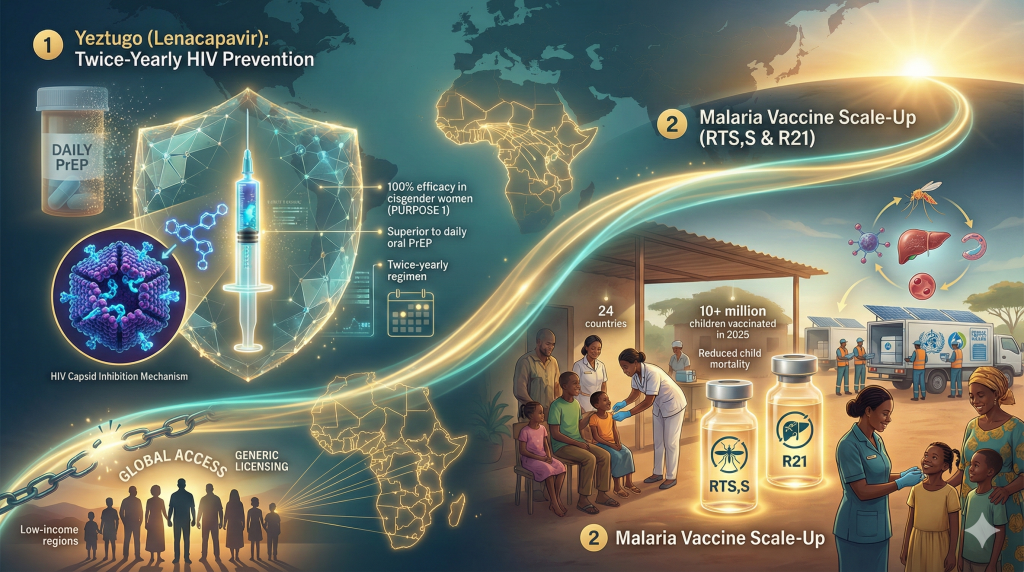
6. Ending Infectious Plagues: Long-Acting Prevention
Infectious disease strategy in 2025 shifted decisively from daily treatment to long-acting prevention, bringing the end of the HIV and Malaria epidemics into view.
Yeztugo (Lenacapavir): The Twice-Yearly Shield
In June 2025, the FDA approved Gilead Sciences’ Yeztugo (lenacapavir) for HIV Pre-Exposure Prophylaxis (PrEP).33
- The Breakthrough: Yeztugo is a capsid inhibitor administered via subcutaneous injection just twice a year.
- Efficacy Data: In the PURPOSE 1 and PURPOSE 2 trials, Yeztugo demonstrated superior efficacy to daily oral Truvada. Notably, in the PURPOSE 1 trial involving cisgender women in Africa, the drug showed 100% efficacy (zero infections), compared to a background incidence rate of 2.41 per 100 person-years.34
- Implication: By removing the burden of daily adherence, Yeztugo effectively neutralizes the behavioral barriers to HIV prevention. The conversation in 2025 shifted to global access, with intense pressure on Gilead to enable generic licensing for low-income nations.36
Malaria Vaccine Scale-Up
2025 also marked the operational zenith of the malaria vaccine rollout. Supported by Gavi and the WHO, the RTS,S/AS01 and R21/Matrix-M vaccines were integrated into the routine immunization schedules of 24 African countries.37 By the end of 2025, over 10 million children had been targeted, a logistical triumph expected to reduce all-cause child mortality significantly in sub-Saharan Africa.37
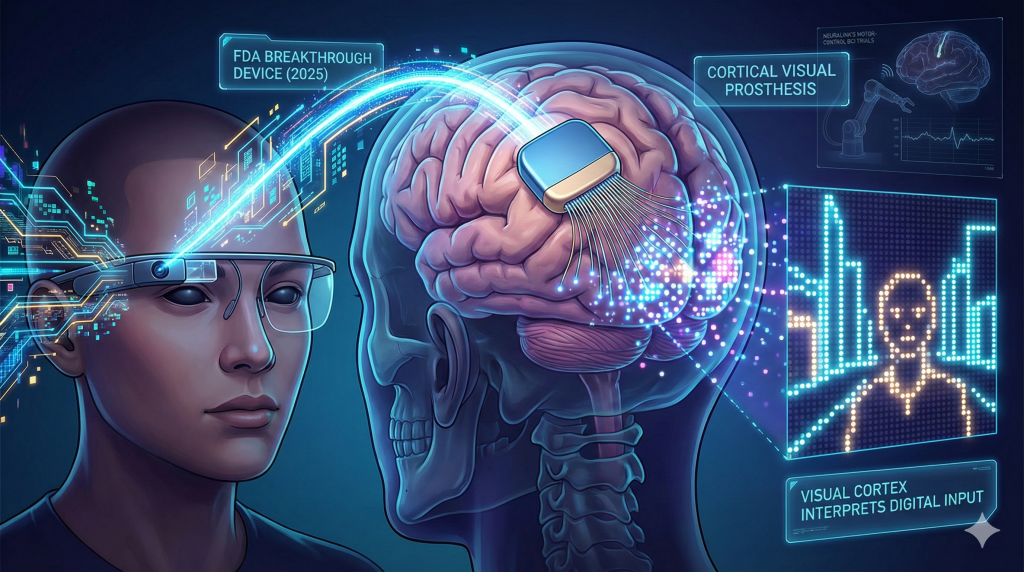
7. Neurotechnology: The Restoration of Senses
The interface between the human brain and digital systems transitioned from concept to regulatory-grade reality in 2025, led by Neuralink.
Blindsight: Bypassing the Eye
Throughout 2025, Neuralink achieved critical regulatory milestones for its Blindsight device, culminating in an FDA Breakthrough Device Designation.39
- Concept: Blindsight is a cortical visual prosthesis. It bypasses the eye and optic nerve entirely, intending to restore vision to individuals who are blind due to ocular or optic nerve damage (e.g., glaucoma, optic atrophy, trauma).
- Mechanism: A camera captures the visual scene, which is processed into a digital signal. This signal is transmitted wirelessly to an N1 implant embedded in the visual cortex. The implant’s microelectrodes stimulate specific neurons to create “phosphenes” (points of light) that the brain interprets as visual data.39
- Status: While early resolution is low (likened by Elon Musk to “Atari graphics”), the biological proof-of-concept—that the visual cortex can accept and interpret digital input—was validated in 2025. Human trials for vision restoration were slated to follow the ongoing “Telepathy” (motor control) trials.41
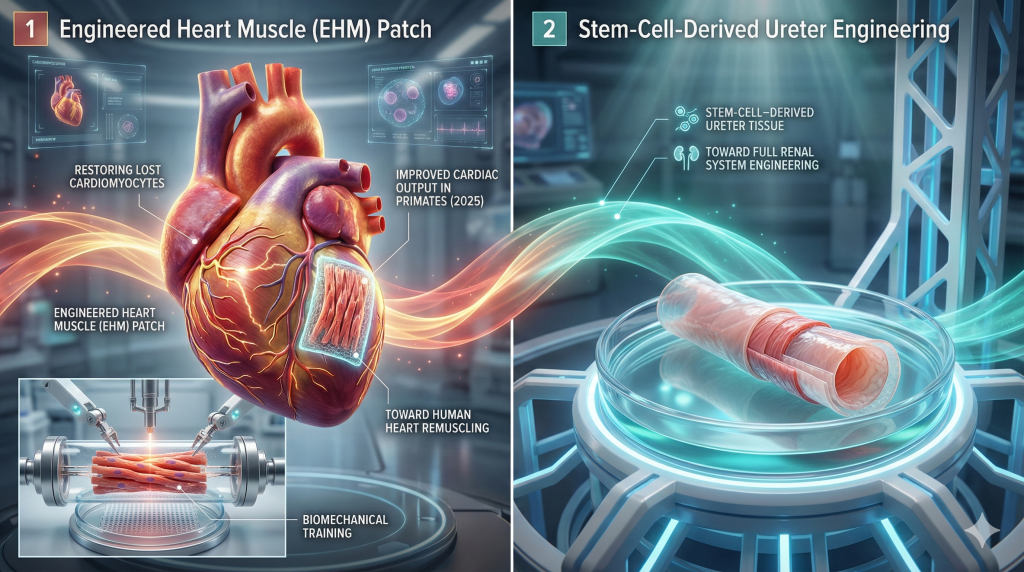
8. Regenerative Engineering: The Heart Patch and Ureter
Regenerative medicine in 2025 moved beyond simple cell injections to the engineering of complex, functional tissues.
The Engineered Heart Muscle (EHM)
Heart failure is often driven by the permanent loss of cardiomyocytes (heart muscle cells) after a heart attack. In a breakthrough published in Nature and advanced into human trials in 2025, researchers at the University Medical Center Göttingen developed an implantable Engineered Heart Muscle (EHM) patch.5
- Bioengineering: The patches are created from induced pluripotent stem cells (iPSCs) differentiated into cardiomyocytes and embedded in a collagen matrix. They are “trained” in the lab to beat synchronously before implantation.
- Primate Data: Crucially, 2025 data showed that these patches could integrate electrically and mechanically with the host heart in rhesus macaques, thickening the heart wall and improving cardiac output without causing arrhythmias—a major failure point of previous cell therapies.43
- Human Application: This technology offers the first potential “remuscling” of the failing human heart, serving as a bridge to recovery or an alternative to transplant.45
Simultaneously, researchers in 2025 successfully created functioning ureter tissue from stem cells, a critical step toward the bioengineering of the complete renal system.5
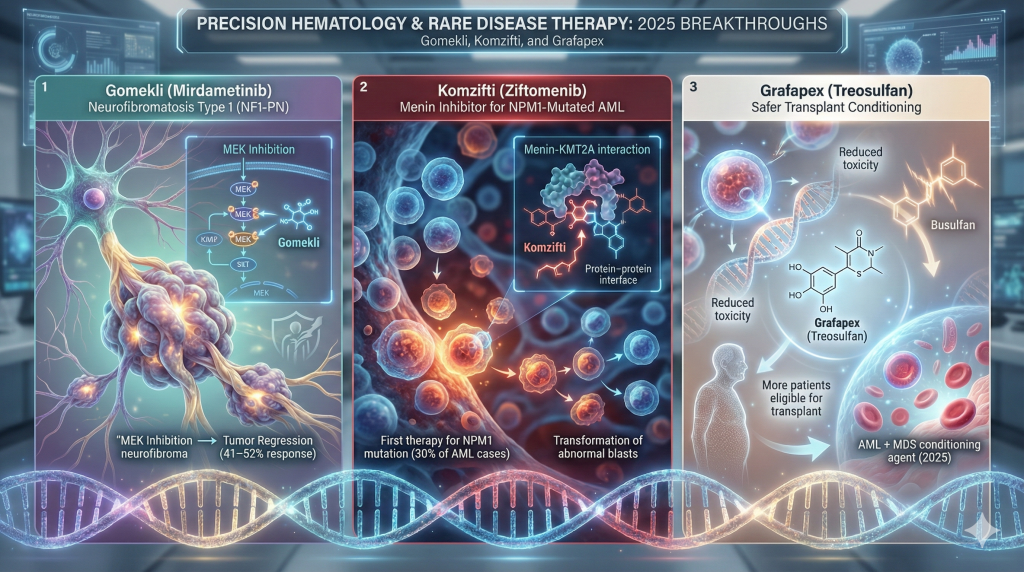
9. Precision Hematology and Orphan Diseases
2025 was a prolific year for “Orphan” conditions—rare diseases that have historically been neglected. The approvals in this space demonstrate the power of understanding specific molecular drivers.
Gomekli (Mirdametinib): Treating NF1
In February 2025, the FDA approved Gomekli (mirdametinib) for Neurofibromatosis Type 1 (NF1) with plexiform neurofibromas (PN).46
- Impact: NF1-PN causes disfiguring and painful tumors that grow along nerves. Gomekli, a MEK inhibitor, demonstrated a 41-52% confirmed response rate in shrinking these tumors, offering a non-surgical option for patients who previously had none.47
Komzifti (Ziftomenib): The Menin Inhibitor for AML
In November 2025, Komzifti (ziftomenib) was approved for relapsed/refractory Acute Myeloid Leukemia (AML) with NPM1 mutations.48
- Mechanism: This drug targets the menin-KMT2A interaction, a key driver of leukemogenesis in NPM1-mutated cells.
- Significance: It is the first therapy specifically approved for the NPM1 mutation, which is the most common genetic alteration in AML (approx. 30% of cases), shifting the standard of care for relapsed patients.50
Grafapex (Treosulfan): Safer Transplants
Also in 2025, Grafapex (treosulfan) was approved as a conditioning agent for stem cell transplants in AML and MDS.51 It offers a less toxic alternative to traditional busulfan-based regimens, potentially expanding transplant eligibility to older patients who could not previously tolerate the procedure.52
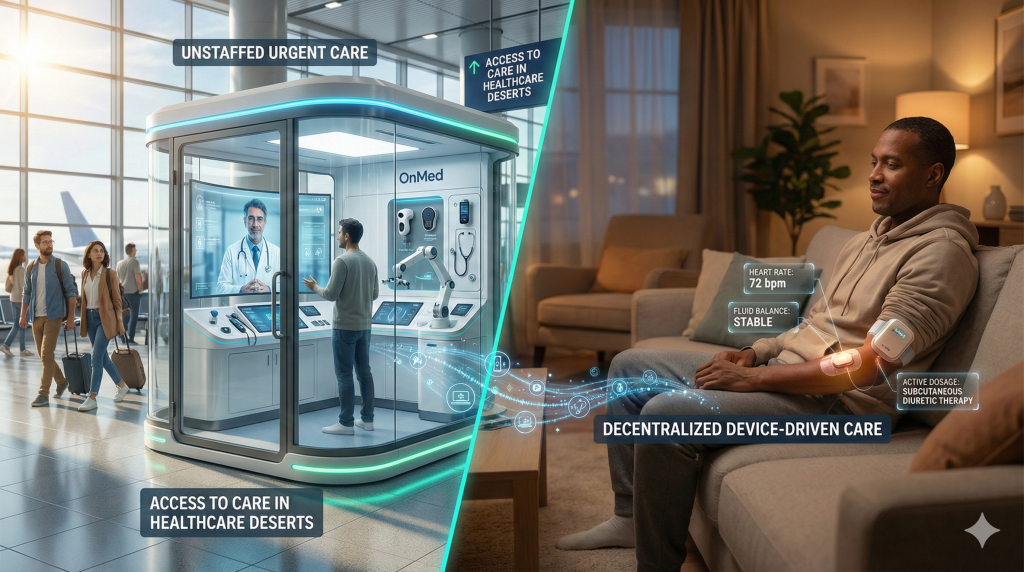
10. Decentralized and Device-Driven Care
The final breakthrough of 2025 is the physical restructuring of care delivery. Facing workforce shortages, the industry pivoted to technology that decouples care from the hospital.
OnMed CareStation: The “Doctor in a Box”
Recognized as one of the best inventions of 2025, the OnMed CareStation is a standalone telehealth pod deployed in airports, universities, and rural centers.53
- Capabilities: Unlike phone-based telehealth, the CareStation features robotic dispensing and medical-grade diagnostics (thermal imaging, stethoscopes, high-res cameras) that allow a remote clinician to perform a physical exam.
- Impact: These units effectively act as unstaffed urgent care centers, plugging gaps in rural healthcare deserts where hospitals have closed.
Lasix ONYU: Hospital Care at Home
In October 2025, the FDA approved Lasix ONYU, a novel on-body infusor for furosemide.26
- Significance: This device allows heart failure patients to receive subcutaneous diuretic therapy at home, a treatment previously requiring intravenous administration in a hospital. This represents a tangible move toward the “Hospital at Home” model, reducing readmission rates for heart failure exacerbations.
Epilogue: Mapping the Future with the Human Cell Atlas
Underpinning many of these breakthroughs was the 2025 publication of the Human Cell Atlas (HCA) comprehensive maps.55 In a series of Nature papers, the HCA consortium released high-resolution, single-cell atlases of the human brain, lung, and developing embryo.56
This achievement is analogous to the Human Genome Project. By mapping every cell type, its location, and its molecular state in health and disease, the HCA is providing the reference map for the next decade of drug discovery. The insights from the developing brain atlas, for instance, are already being used to understand the origins of pediatric neurodevelopmental disorders.
As we look toward 2026, the breakthroughs of 2025—from the atomic precision of gene editing to the macroscopic reorganization of care delivery—signal a future where healthcare is more precise, more curative, and more accessible than ever before.
Summary Data Tables
Table 1: Key FDA Novel Drug Approvals 2025 (Selected)
| Drug Name | Active Ingredient | Indication | Approval Date | Mechanism Class |
| Datroway | Datopotamab deruxtecan | NSCLC / Breast Cancer | Jan/June 2025 | TROP2-directed ADC |
| Journavx | Suzetrigine | Acute Pain | Jan 30, 2025 | NaV1.8 Inhibitor (Non-Opioid) |
| Gomekli | Mirdametinib | NF1-PN | Feb 11, 2025 | MEK Inhibitor |
| Yeztugo | Lenacapavir | HIV PrEP | June 18, 2025 | Capsid Inhibitor (Long-acting) |
| Imdelltra | Tarlatamab | SCLC | Nov 19, 2025 | BiTE (DLL3 x CD3) |
| Komzifti | Ziftomenib | AML (NPM1 mut) | Nov 13, 2025 | Menin Inhibitor |
| Grafapex | Treosulfan | AML/MDS Conditioning | Jan 22, 2025 | Alkylating Agent |
| Avance | Acellular nerve allograft | Nerve Repair | Dec 3, 2025 | Biologic Scaffold |
| Lasix ONYU | Furosemide | Heart Failure Edema | Oct 7, 2025 | On-Body Drug Device |
Table 2: Comparative Efficacy of 2025 Oncology Breakthroughs
| Therapy | Indication | Key Trial Result (vs Control) | Metric | Citation |
| mRNA-4157 + Pembrolizumab | High-Risk Melanoma | 49% reduction in recurrence/death | RFS (Recurrence-Free Survival) | 10 |
| Imdelltra (Tarlatamab) | Extensive-Stage SCLC | 13.6 months vs 8.3 months | Median Overall Survival (mOS) | 21 |
| Datroway (Dato-DXd) | EGFR-mutated NSCLC | 45% confirmed response rate | ORR (Objective Response Rate) | 57 |
| Komzifti (Ziftomenib) | R/R NPM1 AML | 21% complete remission | CR (Complete Remission) | 48 |
Works cited
- Vertex Presents New Data on CASGEVY®, Including First-Ever Data in Children Ages 5-11 Years, at the American Society of Hematology Annual Meeting and Announces Plan for Global Regulatory Submissions, accessed December 10, 2025, https://news.vrtx.com/news-releases/news-release-details/vertex-presents-new-data-casgevyr-including-first-ever-data
- Vertex CRISPR therapy hits early goal in children with blood disorders – BioPharma Dive, accessed December 10, 2025, https://www.biopharmadive.com/news/ASH-Vertex-Casgevy-childen-sickle-cell-thalassemia/807182/
- CRISPR Therapeutics Provides Business Update and Reports Third Quarter 2025 Financial Results, accessed December 10, 2025, https://ir.crisprtx.com/news-releases/news-release-details/crispr-therapeutics-provides-business-update-and-reports-third-6/
- CRISPR Therapeutics Provides First Quarter 2025 Financial Results and Announces Positive Top-Line Data from Phase 1 Clinical Trial of CTX310™ Targeting ANGPTL3, accessed December 10, 2025, https://crisprtx.com/about-us/press-releases-and-presentations/crispr-therapeutics-provides-first-quarter-2025-financial-results-and-announces-positive-top-line-data-from-phase-1-clinical-trial-of-ctx310-targeting-angptl3
- 9 medical breakthroughs that gave us hope in 2025 | National Geographic, accessed December 10, 2025, https://www.nationalgeographic.com/health/article/top-health-discoveries-of-2025
- World’s First Patient Treated with Personalized CRISPR Gene Editing Therapy at Children’s Hospital of Philadelphia, accessed December 10, 2025, https://www.chop.edu/news/worlds-first-patient-treated-personalized-crispr-gene-editing-therapy-childrens-hospital
- CRISPR Clinical Trials: A 2025 Update – Innovative Genomics Institute (IGI), accessed December 10, 2025, https://innovativegenomics.org/news/crispr-clinical-trials-2025/
- Baby KJ’s CRISPR treatment: Family update, researcher’s next steps, accessed December 10, 2025, https://nucdf.org/news.html/article/2025/11/23/baby-kj-s-crispr-treatment-family-offers-update-researchers-share-next-steps
- Researchers Behind Personalized CRISPR Therapy Plan to Launch a New Type of Clinical Trial Specifically for Rare Diseases | Children’s Hospital of Philadelphia, accessed December 10, 2025, https://www.chop.edu/news/researchers-behind-personalized-crispr-therapy-plan-launch-new-type-clinical-trial
- Moderna and Merck Announce New 3-Year Data for mRNA-4157 Combined With Pembrolizumab for High-Risk Stage III/IV Melanoma | Dermatology Times, accessed December 10, 2025, https://www.dermatologytimes.com/view/moderna-and-merck-announce-new-3-year-date-for-mrna-4157-combined-with-pembrolizumab-for-high-risk-stage-iii-iv-melanoma
- A Clinical Trial of a Personalized Cancer Vaccine for Adults with High-Risk Melanoma, accessed December 10, 2025, https://trials.modernatx.com/study/?id=mRNA-4157-P201
- Moderna and MSD share encouraging three-year data from trial of melanoma therapy, accessed December 10, 2025, https://www.clinicaltrialsarena.com/news/moderna-msd-melanoma-trial/
- mRNA medicines we are currently developing – Moderna, accessed December 10, 2025, https://www.modernatx.com/research/product-pipeline?
- Merck and Moderna Initiate Phase 3 Trial Evaluating Adjuvant V940 (mRNA-4157) in Combination with KEYTRUDA® (pembrolizumab) After Neoadjuvant KEYTRUDA and Chemotherapy in Patients With Certain Types of Non-Small Cell Lung Cancer (NSCLC), accessed December 10, 2025, https://www.merck.com/news/merck-and-moderna-initiate-phase-3-trial-evaluating-adjuvant-v940-mrna-4157-in-combination-with-keytruda-pembrolizumab-after-neoadjuvant-keytruda-and-chemotherapy-in-patients-with-certain-ty/
- FDA approves datopotamab deruxtecan-dlnk for unresectable or metastatic, HR-positive, HER2-negative breast cancer, accessed December 10, 2025, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-datopotamab-deruxtecan-dlnk-unresectable-or-metastatic-hr-positive-her2-negative-breast
- FDA grants accelerated approval to datopotamab deruxtecan-dlnk for EGFR-mutated non-small cell lung cancer, accessed December 10, 2025, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-datopotamab-deruxtecan-dlnk-egfr-mutated-non-small-cell-lung-cancer
- Datopotamab deruxtecan – Wikipedia, accessed December 10, 2025, https://en.wikipedia.org/wiki/Datopotamab_deruxtecan
- DATROWAY® (datopotamab deruxtecan-dlnk) demonstrated an unprecedented median overall survival improvement of five months vs. chemotherapy as 1st-line treatment for patients with metastatic triple-negative breast cancer for whom immunotherapy was not an option in TROPION-Breast02 – AstraZeneca US, accessed December 10, 2025, https://www.astrazeneca-us.com/media/press-releases/2025/DATROWAY-datopotamab-deruxtecan-dlnk-demonstrated-an-unprecedented-median-overall-survival-improvement-of-five-months-vs-chemotherapy-as-1st-line-treatment-for-patients-with-metastatic-triple-negative-breast-cancer-for-whom-immunotherapy-was-not-an-option-in-TROPION-Breast02.html
- DATROWAY® Approved in the U.S. as First TROP2 Directed Therapy for Patients with Previously Treated Advanced EGFR-Mutated Non-Small Cell Lung Cancer – Daiichi Sankyo, accessed December 10, 2025, https://daiichisankyo.us/press-releases/-/article/datroway-approved-in-the-us-as-first-trop2-directed-therapy-for-patients-with-previously-treated-advanced-egfr-mutated-non-small-cell-lung-cancer
- FDA grants traditional approval to tarlatamab-dlle for extensive stage small cell lung cancer, accessed December 10, 2025, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-traditional-approval-tarlatamab-dlle-extensive-stage-small-cell-lung-cancer
- FDA Grants Full Approval to Tarlatamab for Extensive-Stage Small Cell Lung Cancer, accessed December 10, 2025, https://www.onclive.com/view/fda-grants-full-approval-to-tarlatamab-for-extensive-stage-small-cell-lung-cancer
- IMDELLTRA® DEMONSTRATED SUPERIOR OVERALL SURVIVAL IN SMALL CELL LUNG CANCER – PR Newswire, accessed December 10, 2025, https://www.prnewswire.com/news-releases/imdelltra-demonstrated-superior-overall-survival-in-small-cell-lung-cancer-302426291.html
- FDA GRANTS FULL APPROVAL TO AMGEN’S IMDELLTRA® IN EXTENSIVE STAGE SMALL CELL LUNG CANCER, accessed December 10, 2025, https://www.amgen.com/newsroom/press-releases/2025/11/fda-grants-full-approval-to-amgens-imdelltra-in-extensive-stage-small-cell-lung-cancer
- accessed December 10, 2025, https://en.wikipedia.org/wiki/Suzetrigine
- Journavx: Uses, Dosage, Side Effects, Warnings – Drugs.com, accessed December 10, 2025, https://www.drugs.com/journavx.html
- New FDA Drug Approvals for 2025 – Drugs.com, accessed December 10, 2025, https://www.drugs.com/newdrugs.html
- 3 Health Care Takeaways from Google I/O 2025 | AHA, accessed December 10, 2025, https://www.aha.org/aha-center-health-innovation-market-scan/2025-06-03-3-health-care-takeaways-google-io-2025
- Advancing Cutting-edge AI Capabilities – Google for Health, accessed December 10, 2025, https://health.google/ai-models
- Google Research at Google I/O 2025, accessed December 10, 2025, https://research.google/blog/google-research-at-google-io-2025/
- New Dataset Makes Health Chatbots Like Google’s MedGemma More Mindful of African Contexts | College of Computing – Georgia Institute of Technology, accessed December 10, 2025, https://www.cc.gatech.edu/news/new-dataset-makes-health-chatbots-googles-medgemma-more-mindful-african-contexts
- Advancing healthcare and scientific discovery with AI – Google Blog, accessed December 10, 2025, https://blog.google/technology/health/google-research-healthcare-ai/
- 8 Best Healthcare Inventions of 2025, According to TIME: AI, Automation and Access – Xtalks, accessed December 10, 2025, https://xtalks.com/8-best-healthcare-inventions-of-2025-according-to-time-ai-automation-and-access-4441/
- Yeztugo® (lenacapavir) is now the first and only FDA-approved HIV prevention option offering 6 months of protection, accessed December 10, 2025, https://www.eatg.org/hiv-news/yeztugo-lenacapavir-is-now-the-first-and-only-fda-approved-hiv-prevention-option-offering-6-months-of-protection/
- A New Twice-Yearly PrEP: What Is Yeztugo? – Mount Sinai Today, accessed December 10, 2025, https://health.mountsinai.org/blog/a-new-twice-yearly-prep-what-is-yeztugo/
- Yeztugo® (Lenacapavir) Is Now the First and Only FDA Approved HIV Prevention Option Offering 6 Months of Protection – Gilead Sciences, accessed December 10, 2025, https://www.gilead.com/news/news-details/2025/yeztugo-lenacapavir-is-now-the-first-and-only-fda-approved-hiv-prevention-option-offering-6-months-of-protection
- 6 things to know about lenacapavir, what experts call a ‘wonder drug’ for preventing HIV, accessed December 10, 2025, https://www.pbs.org/newshour/health/6-things-to-know-about-lenacapavir-what-experts-call-a-wonder-drug-for-preventing-hiv
- accessed December 10, 2025, https://www.who.int/news-room/questions-and-answers/item/q-a-on-rts-s-malaria-vaccine#:~:text=By%20the%20close%20of%202025,international%20and%20country%2Dlevel%20partners.
- Malaria vaccines (RTS,S and R21) – World Health Organization (WHO), accessed December 10, 2025, https://www.who.int/news-room/questions-and-answers/item/q-a-on-rts-s-malaria-vaccine
- Visual Prosthesis | Neuralink, accessed December 10, 2025, https://neuralink.com/trials/visual-prosthesis/
- Neuralink’s Blindsight granted US FDA’s Breakthrough Device Designation, accessed December 10, 2025, https://www.medicaldeviceszone.com/neurology/neuralinks-blindsight-granted-us-fdas-breakthrough-device-designation/
- Neuralink to begin human trials for vision implant by 2026 – Perplexity, accessed December 10, 2025, https://www.perplexity.ai/page/neuralink-to-begin-human-trial-AbI5KecnTP.DBZMW.hIy7w
- DZHK research paves the way for ‘heart patch’ therapy in heart failure, accessed December 10, 2025, https://dzhk.de/en/newsroom/news/latest-news/article/dzhk-studie-ebnet-weg-fuer-herzpflaster-therapie-bei-herzschwaeche
- ‘Groundbreaking’: scientists develop patch that can repair damaged hearts – The Guardian, accessed December 10, 2025, https://www.theguardian.com/science/2025/jan/29/scientists-develop-patch-repair-damage-heart-failure
- Animal studies confirm the safety and effectiveness of the heart patch | DPZ, accessed December 10, 2025, https://www.dpz.eu/en/public-engagement/news/article/tierversuche-bestaetigen-sicherheit-und-wirksamkeit-des-herzpflasters
- Scientists develop implantable patch to repair damaged hearts | The Business Standard, accessed December 10, 2025, https://www.tbsnews.net/bangladesh/health/scientists-develop-implantable-patch-repair-damaged-hearts-1079666
- accessed December 10, 2025, https://www.gomekli.com/#:~:text=GOMEKLI%20(mirdametinib)%20is%20a%20prescription,be%20completely%20removed%20by%20surgery.
- FDA approves mirdametinib for adult and pediatric patients with neurofibromatosis type 1 who have symptomatic plexiform neurofibromas not amenable to complete resection, accessed December 10, 2025, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-mirdametinib-adult-and-pediatric-patients-neurofibromatosis-type-1-who-have-symptomatic
- FDA Approves Ziftomenib for NPM1+ R/R Acute Myeloid Leukemia | OncLive, accessed December 10, 2025, https://www.onclive.com/view/fda-approves-ziftomenib-for-npm1-r-r-acute-myeloid-leukemia
- FDA approves ziftomenib for relapsed or refractory acute myeloid leukemia with a NPM1 mutation, accessed December 10, 2025, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ziftomenib-relapsed-or-refractory-acute-myeloid-leukemia-npm1-mutation
- Kura Oncology and Kyowa Kirin Announce FDA Approval of KOMZIFTI™ (ziftomenib), the First and Only Once-Daily Targeted Therapy for Adults with Relapsed or Refractory NPM1-Mutated Acute Myeloid Leukemia, accessed December 10, 2025, https://ir.kuraoncology.com/news-releases/news-release-details/kura-oncology-and-kyowa-kirin-announce-fda-approval-komziftitm
- accessed December 10, 2025, https://www.grafapex.com/#:~:text=Acute%20Myeloid%20Leukemia%3A%20GRAFAPEX%20is,old%20with%20acute%20myeloid%20leukemia.
- GRAFAPEX (treosulfan) for Injection, accessed December 10, 2025, https://www.grafapex.com/
- OnMed CareStation: The Best Inventions of 2025 – Time Magazine, accessed December 10, 2025, https://time.com/collections/best-inventions-2025/7318449/onmed-carestation/
- OnMed Named to TIME’s List of the Best Inventions of 2025, accessed December 10, 2025, https://www.onmed.com/resources/onmed-named-to-times-list-of-the-best-inventions-of-2025
- Publications – Human Cell Atlas, accessed December 10, 2025, https://www.humancellatlas.org/publications/
- Scientists complete first drafts of developing mammalian brain cell atlases – Allen Institute, accessed December 10, 2025, https://alleninstitute.org/news/scientists-complete-first-drafts-of-developing-mammalian-brain-cell-atlases/
- DATROWAY® Approved in the US as First TROP2 Directed Therapy for Patients with Previously Treated Advanced EGFR- Mutated Non-Small Cell Lung Cancer – Daiichi Sankyo, accessed December 10, 2025, https://www.daiichisankyo.com/files/news/pressrelease/pdf/202506/20250624_E.pdf
Study results open door to heart failure treatment with ‘heart patch’ – Universitätsmedizin Göttingen, accessed December 10, 2025, https://www.umg.eu/en/news-detail/news-detail/detail/news/study-results-open-door-to-heart-failure-treatment-with-heart-patch/


